

But This.
Sodium acetate or hot ice is an amazing chemical you can prepare from baking soda and vinegar. Cooling of sodium acetate solution below its melting point will cause the liquid to crystallize. The crystallization is an exothermic process, so the resulting ice is hot.
Alternatively, Sodium thiosulphate-5-water could be used.
Apparatus and chemicals:
Boiling Tube
Stirring Thermometer
Beaker (100cm3)
Bunsen Burner
Tripod and wire gauze
Sodium acetate crystals
Hows its done:
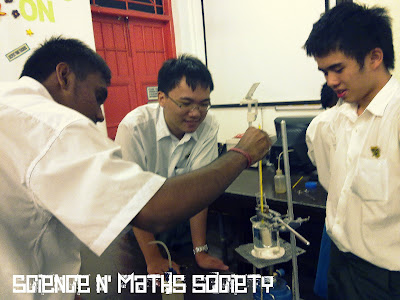
The apparatus is set up and sodium acetate is left to melt in a water bath into a clear liquid. This process takes about 20-30minutes for the water to vapourise completely.
The sodium acetate solution is then left to cool in a beaker of iced water.
(If the liquid does start to crystallise,it is reheated to melt all the solid once more.)
Observe that at 30-40 degree Celcius, crystallization should not occur.
Add a fresh crystal of sodium acetate (drop it in the solution)and observe rapid crystallization occuring.
:) TADAA

Setting the apparatus up.

Reo Sei adjusting the height :)
The meeting ended about 4.45pm. Most of our sodium acetate crystallized before we even touch it, but ofcourse, some succeeded in this experiment too. The main key is to cover the boiling tube to prevent foreign particles to enter and freeze it.
And yes, the ice was burning hot.
:D

No comments:
Post a Comment